Abstract
Introduction: To date, the ELN recommendation and NCCN guidelines are the principle mile stones to follow up the treatment response and to make the decision of TKIs switching. However, in real life practice, many factors influence changing the real switching date from the date had an indication. This study aims to analyze the impact of early switching to second line TKI, nilotinib, in real life practice, for the CML patients who failed, had sub-optimal response or were intolerant to imatinib.
Methods: This prospective study was conducted through 7 medical centers in Thailand between 1st of September 2009 and 31st of August 2011. Adult CML patients of age ≥ 18 years old, in chronic and accelerated phase, who had failure, suboptimal response or intolerance to imatinib, based on ELN 2009 guideline, were included and were eligible with nilotinib 400 mg twice daily. Prospective data collection for 24 months of each patient was performed. The main objective was to identify the impact of early switching to nilotinib on major molecular response (MMR). The other objectives were to observe the efficacy of nilotinib including overall survival, progression free survival and the safety. The survival results were presented as Kaplan-Meier survival curves. For the comparison of the treatment groups, the Kaplan-Meier estimator with the corresponding log-rank test for equality of survivor functions across treatment group was applied.
Results: The final 108 cases were analysed. The median age was 47 (17-79) years with the proportion of male to female of 1.4:1 respectively. The median duration of the prior imatinib treatment was 18 months (2-142 months). The median duration between the date of indication and the date of real switching was 3.1 months (0-62.8 months) with 50% changing less than 3 months, 26.9% between 3 months and 12 months, and 23.1% changing longer than 12 months. The indication of switching included 63.6% failure to imatinib, 29% intolerance to imatinib and 7.4% suboptimal to imatinib. On the nilotinib switching, 70.4% completed 24 months follow-up, and 29.6% discontinued treatment mostly because of unsatisfactory results or adverse events.
Evaluation was made every 3 months based on 2009 ELN recommendation. At 3 months, 57%, 20%, and 8% of the patients achieved CHR, CCyR and MMR, respectively. Those who did not achieve CHR at 3 months never achieved MMR, while 86 % of those who achieved CCyR at 3 months achieved MMR. All CML achieving MMR at 3 months had sustained MMR throughout the study period (24 months). Imatinib suboptimal response had better outcome than imatinib failure and imatinib intolerance groups.
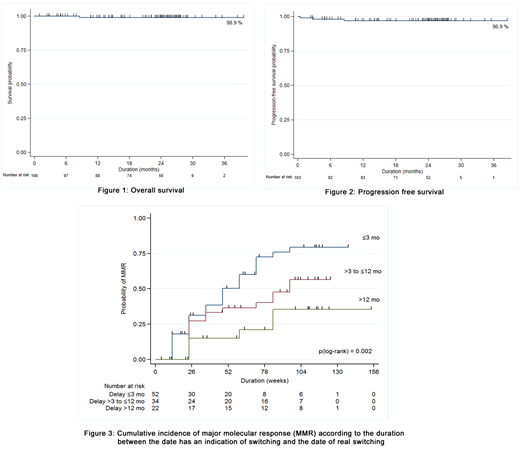
A preliminary analysis of BCR-ABL mutation was performed on 90 cases, and mutations were found on 21 cases. Two of them were T315I which were excluded from the study. The cases with mutation had poorer response to treatment than those without mutation. There was one case with initial G250E mutation developing T315I mutation after treatment with nilotinib. At 24 months, one case progressed to accelerated phase and 3 cases progressed to blastic transformation. The 2-year overall survival and 2-year progression-free survival and were 98.9% and 96.9% (figure 1 and 2), respectively. The interquatile analysis was done to identify the groups of cumulative MMR according to the duration between the date of indication and the date of real switching to nilotinib. The patients who switched to nilotinib within 12 months after date of indication could have a greater chance to achieved MMR than those who switched treatment later than 12 months (p(log-rank) = 0.002) (figure 3).
Skin rash, musculoskeletal pain, and infection were the three most common non-hematologic adverse events, However, most of them were grade 1-2, except for 4 cases with grade 3-4 infections. Grade 3-4 hematologic adverse events included thrombocytopenia (12%), neutropenia (11%), anemia (5%) and leucopenia (4%), and most of them were manageable. Although biochemical abnormalities were commonly found, most of them were mild.
Conclusions: Nilotinib, as a second line treatment showed excellent efficacy and tolerability. Indication for nilotinib treatment, initial mutation status and depth of response at 3 months after treatment can predict outcomes of the patients. However, the patients will have a greater chance to achieve MMR if they switched to nilotinib within 12 months after the date of indication for changing.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal